Study Design
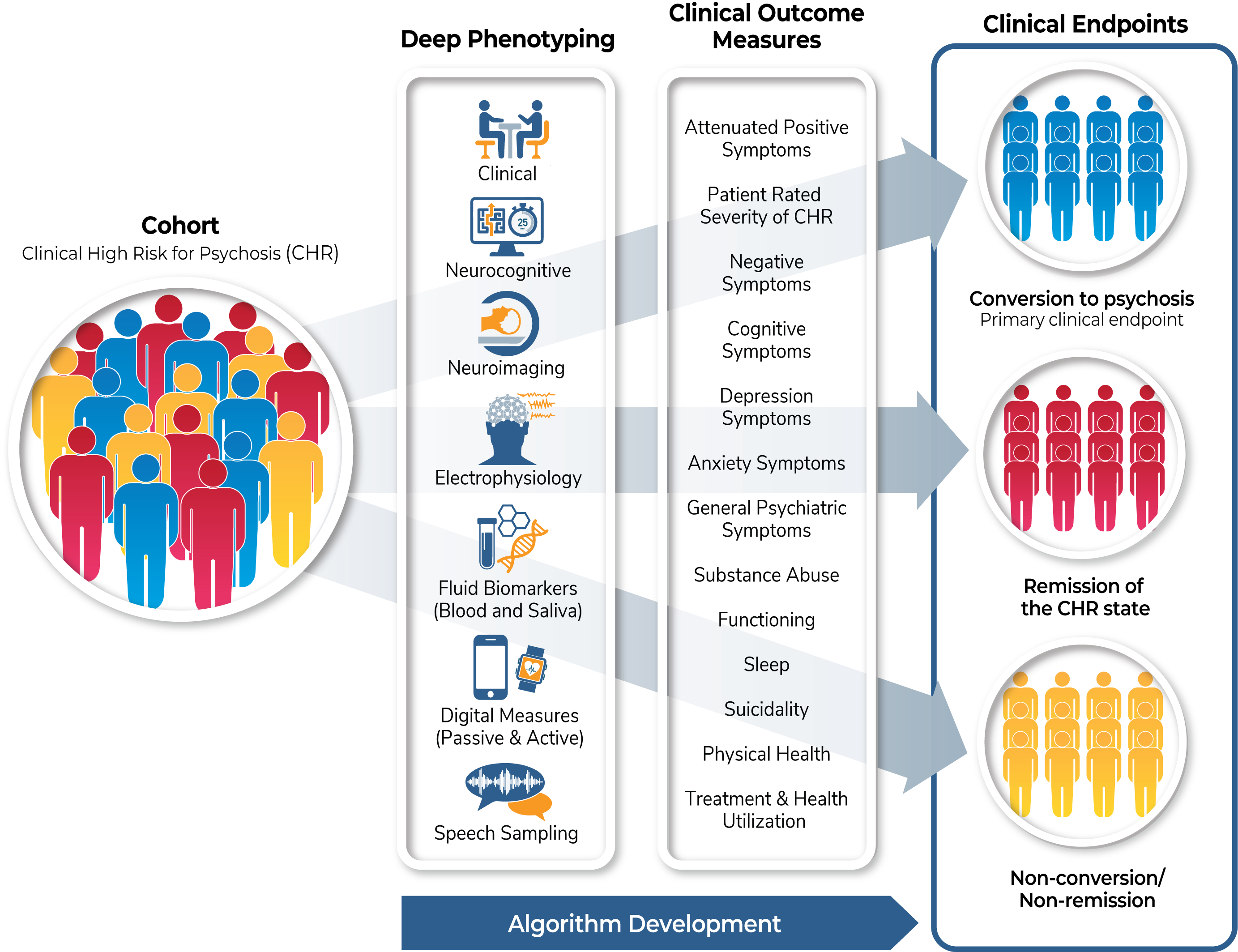
This is a non-interventional study examining clinical trajectories and predictors of outcomes in the clinical high risk (CHR) population.
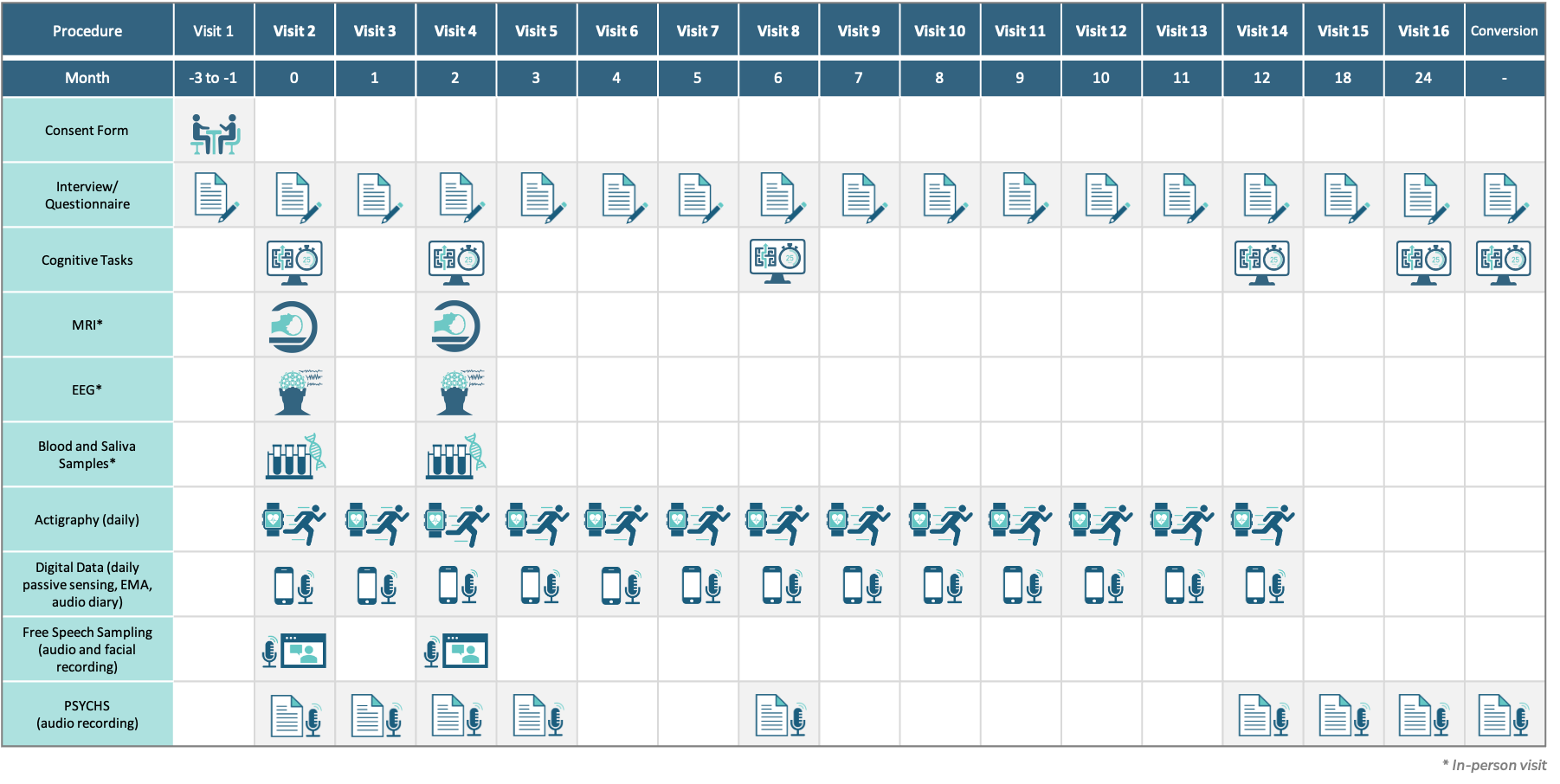
Clinical assessments will be collected monthly for the first year, and then at 18 and 24 months and at the point of conversion to psychosis.
The biomarker measures (imaging, EEG and event-related potentials, fluid-based biomarkers, cognitive assessments, and speech sampling) will be collected at baseline and at two months after study entry. Digital assessments (actigraphy as well phone app-based data collection) will be collected daily for the first year. The collection of these biomarkers over time will validate their use and efficacy in the CHR population to establish early indicators of pharmacologic treatment efficacy.
Cognitive assessments will be collected longitudinally at six, 12 and 24 months and at conversion to psychosis. Unbiased machine language and AI approaches will be used to derive algorithms that predict clinical endpoints: conversion to psychosis (primary clinical endpoint); remission of the CHR state (secondary clinical endpoint), and non-conversion/non-remission (secondary clinical endpoint). These approaches will allow the selection of enriched populations to help improve success in developing pharmacologic treatments.
The goal of AMP® SCZ is to facilitate drug development for the CHR population.
Last Reviewed on December 20, 2022